Boltzmann Maps is pleased to introduce an integration with Pharmit as an option for pharmacophore screening. Pharmit is a search tool for finding small molecule inhibitors that bind to a target of interest. The tool searches libraries for compounds with desired features in the right geometry. Boltzmann Maps integration allows the user to send a protein-ligand system from BMaps to Pharmit for search based on the compound’s features or other user-specified features. Pharmit’s nine built-in libraries include almost 250M compound entries, and the 1,059 publicly accessible user-contributed libraries contain another 45M entries.
Pharmit can be accessed via the Export button on the bottom right of the BMaps web app.


To stage a pharmacophore search from BMaps, start by loading a protein and then sketching or uploading any compounds with desired features. Then click the Export button, go to the Pharmit Screening tab, and click “Export to Pharmit.” Once in Pharmit, pharmacophore features can be added as a query format or the features can be extracted directly from the ligand. If the receptor loaded already has the small molecule bound, the features of the small molecule interacting with the receptor are retained. Shape search includes a shape filter or pharmacophore filter and the compound is searched against the chosen library of compounds. Under the ‘shape’ section, the user can specify shape constraints to use; either the entire ligand or certain spheres can be excluded or included. Hits can be filtered by conformation, molecule, total hits, molecular weight, rotatable bonds, LogP, polar surface area, aromatics, hydrogen bond acceptor, or hydrogen bond donors.
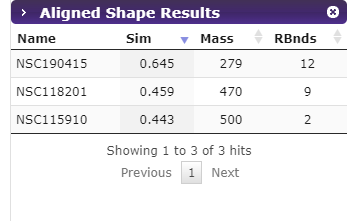
The pharmacophore results contain the compound ID, RMSD, mass, and the number of rotatable bonds.